
It is possible that the messenger RNA SARS-CoV-2 Moderna and Pfizer vaccines can trigger a T cell-mediated immune response with the downstream effects of alopecia.

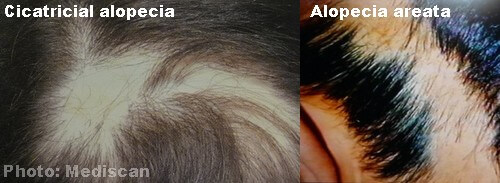
An antibody-mediated response prompted by vaccination may cross-react with self-antigen, leading to autoimmunity. Vaccines have been implicated as triggers of autoimmune disease in genetically predisposed individuals. Patches of nonscarring alopecia with 70% loss of scalp hair sparse eyebrows and eyelashes
#Alopecia universalis vs alopecia areata trial
Pending possible trial of oral tofacitinib citrate Two patches of nonscarring alopecia of the scalp Grandmother with Hashimoto thyroiditis, sister with elevated thyroid antibody Patches of nonscarring alopecia 30% hair loss from scalp, 80% hair loss from beard Two patches of nonscarring alopecia of the scalp with areas of regrowthĮlevated thyroid antibody, normal thyroid function tests Tofacitinib citrate 10 mg twice a day, bimatoprost 0.03% eye dropsĮlevated levels of thyroglobulin antibody and thyroid peroxidase antibody Widespread nonscarring alopecia of the scalp with foci of hair regrowthĬompounded tofacitinib 2%, clobetasol 0.05% ointment, clobetasol solution ILTAC, pimecrolimus 1% cream, clobetasol 0.05% foam Large patches of nonscarring alopecia of the scalp with foci of hair regrowth Hepatic steatosis, chronic hepatitis B virus King.Approximate length of time to flare after vaccine “Hopefully, in the next year people with severe alopecia areata will have yet another treatment option,” says Dr. King was the PI for the clinical trial for ritlecitinib published in The Lancet in April 2023.Ĭlinical trials for another JAK inhibitor, deuruxolitinib, for alopecia areata treatment have also shown positive results. Whereas baricitinib is approved for use in adults, ritlecitinib is approved for use in people ages 12 years and older. In June 2023, the FDA approved ritlecitinib (marketed as LITFULO®), a JAK inhibitor manufactured by Pfizer, for people with severe alopecia areata. In June 2022, the Food and Drug Administration (FDA) approved baricitinib for the treatment of severe alopecia areata in adults. King was the principal investigator (PI) for the trials, which demonstrated that baricitinib helped many patients to regrow hair. One company, Eli Lilly, published the results of two Phase 3 clinical trials for a JAK inhibitor called baricitinib (marketed as Olumiant®) in May 2022 in The New England Journal of Medicine. In 2013, he recognized the potential of a class of medicines called Janus kinase (JAK) inhibitors to treat alopecia areata and subsequently demonstrated their potential for the treatment of alopecia areata and other dermatologic diseases. However, this treatment is often uncomfortable for the patient, causing redness and itchiness.įor more severe cases of alopecia areata, Dr. The resulting inflammation seems to subvert the immune system’s attack on the hair follicles.

Steroids suppress the immune cells that are attacking hair follicles, so hair can regrow.Īnother approach is the topical application of an irritant such as squaric acid, which results in a rash similar to poison ivy. Traditional treatments for alopecia areata include steroids that are either injected or applied directly (creams or liquids) to the areas where the hair has been shed. In cases of relatively mild alopecia areata, meaning there is a limited amount of hair loss, hair may regrow without treatment, although hair loss often recurs.


 0 kommentar(er)
0 kommentar(er)
